On April 20th, the team for molecular target discovery and green pesticide creation at our college published a research paper titled "Suzuki-Miyaura Cross-Coupling of 2-Pyridyl Trimethylammonium Salts by N-C Activation Catalyzed by Air- and Moisture-Stable Pd-NHC Precatalysts: Application to the Discovery of Agrochemicals" online in Organic Letters. Yuge Hu, a master's student, is the first author of the paper, and Associate Professor Peng Lei and Professor Szostak Michal from Rutgers University are the corresponding authors.
Pyridine is one of the most important structures in the creation of new green pesticides. The pyridine ring has good stability and absorption conductivity, which can effectively enhance the pesticide activity of compounds. The nitrogen atom in the pyridine structure contains lone pair electrons, which can form hydrogen bonds or π-π stacking interactions with amino acids in the target protein, and is beneficial for improving the selectivity of the compound and reducing its toxicity.
2-Arylpyridine compounds have excellent pesticide activity, and this study reports an efficient synthesis method for 2-arylpyridine and its application in the creation of green pesticides. The study optimized the best reaction conditions, and demonstrated that the method has excellent substrate compatibility, allowing for the preparation of structurally diverse 2-arylpyridine compounds, compatible with substituted or unsubstituted benzene, thiophene, furan, pyridine, pyrimidine, pyrrole, pyrazole, isoxazole, benzothiophene, benzofuran, oxanthrene, indole, and quinoline, etc (Figure 1). In addition, the study conducted preliminary research on the reaction mechanism through intermolecular competition experiments, attempted in situ generation of ammonium salts from dimethylamine for carbon-nitrogen bond activation synthesis of 2-arylpyridine compounds using a one-pot approach, and multi-step continuous reactions such as carbon-halogen coupling, nitrogen alkylation, and carbon-nitrogen activation, further demonstrating the practicality of this method in drug synthesis.
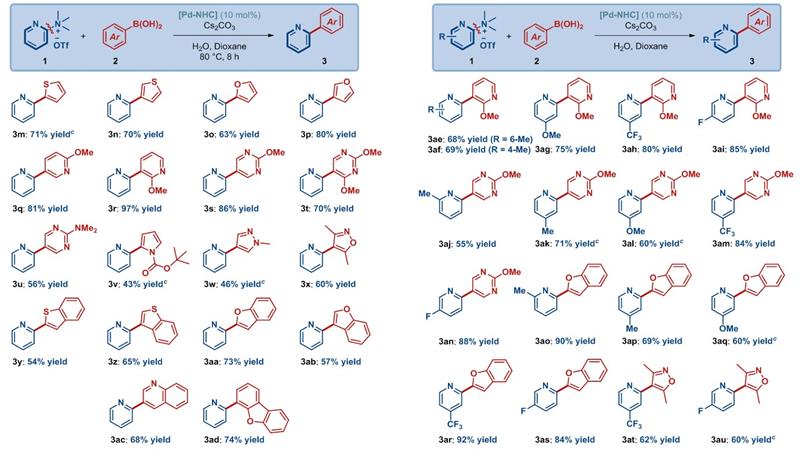
Figure 1: Substrate scope.
Through a "skeleton jumping" approach, 2-pyridine compounds containing indole were designed and synthesized. This compound exhibited good antibacterial activity against pathogens such as rice blast, tomato gray mold, wheat fusarium, apple rot, and tomato early blight (Table 1). This study provides an efficient synthesis method for the preparation of new 2-arylpyridine pesticides, which can improve the efficiency of discovering new 2-arylpyridine pesticides.
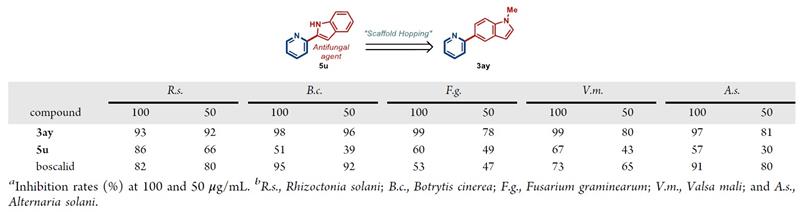
Table 1: Antibacterial activity of compound 3ay.
Professors Liu Xili, Ma Zhiqing, and Feng Juntao, and Associate Professor Yanqing, Gao from our college made important contributions to this work. This research was supported by the Shaanxi Key Research and Development Program and the National Natural Science Foundation of China.
Full text: https://pubs.acs.org/doi/10.1021/acs.orglett.3c00741